Background: Acalabrutinib (acala), a second-generation Bruton tyrosine kinase inhibitor (BTKi), with or without obinutuzumab (obin), demonstrated superior progression-free survival (PFS) compared with obin plus chlorambucil in patients with treatment-naive (TN) chronic lymphocytic leukemia (CLL) in the phase 3 ELEVATE-TN (NCT02475681) trial. Despite the long-term benefits observed with acala therapy, data from real-world studies in patients with CLL demonstrate that 22% of patients discontinue acala treatment for any reason in the first year of treatment across therapy lines (Roeker et al. Blood. 2022). To assess the impact on survival outcomes of acala discontinuation and dose modifications occurring in the first year of treatment, we conducted a post hoc analysis in patients treated in the acala-containing arms of ELEVATE-TN.
Methods: Patients who received acala 100 mg twice daily with or without obin (fixed duration, up to 6 cycles) with at least 1 year of follow-up were included in this analysis. The impact of dose reductions, treatment breaks, or treatment discontinuations in the first year on overall survival (OS) was assessed. Protocol-specified dose-modification guidelines that included dose reductions, breaks, and discontinuations depending on severity and frequency were applied to patients with apparent treatment-related toxicity. To assess the impact of dose reductions, breaks, or discontinuations, patients were divided into 4 dose-modification subgroups: 1) patients with no dose reductions/breaks >14 days during the first year; 2) patients with dose reductions >14 days, but no breaks >14 days during the first year; 3) patients with breaks >14 days, but who continued treatment in the first year; and 4) patients who discontinued treatment during the first year.
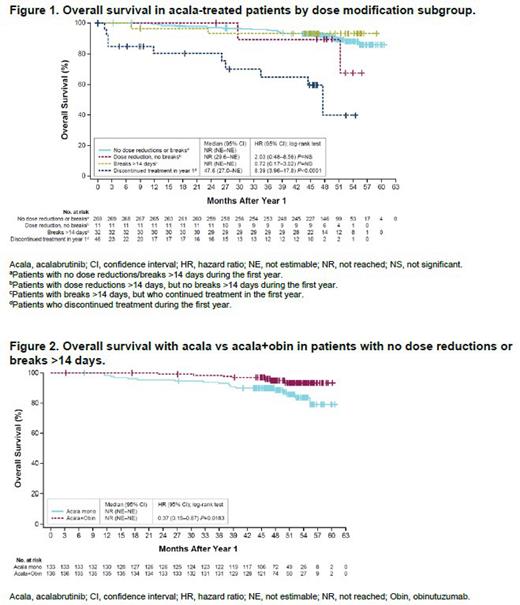
Results: With a median follow-up of 60 months, 358 patients (n=179, acala monotherapy; n=179, acala-obin) with ≥1 year of follow-up were included in this analysis. In the first year, 269 (75%) patients had no dose reductions/breaks >14 days, 11 (3%) patients had dose reductions >14 days but no breaks >14 days, 32 (9%) patients had breaks >14 days but continued treatment, and 46 (13%) patients discontinued treatment. Reasons for treatment discontinuation during the first year were adverse events (n=26), disease progression (n=7), physician decision (n=5), consent withdrawn (n=3), death (n=2), loss to follow-up (n=1), and other (n=2). Patients who discontinued treatment within the first year had inferior OS compared with patients who did not require dose reductions or breaks (OS hazard ratio [HR]: 8.39, 95% confidence interval [CI]: 3.96-17.8, P<0.0001; Figure 1). In contrast, patients who had dose reductions or breaks >14 days but did not discontinue treatment had statistically similar OS to patients who did not require dose reductions or breaks. Within the group that had no dose reductions or treatment breaks >14 days, there was no difference in the proportion of patients treated with acala monotherapy (n=133) and acala-obin (n=136). However, OS outcomes in this subgroup of patients with no treatment modifications were superior in patients treated with acala-obin compared with those treated with acala monotherapy (HR: 0.37, 95% CI 0.15-0.87, P=0.0183; Figure 2).
Conclusions: This post hoc analysis of acala-treated patients with TN CLL in ELEVATE-TN demonstrated superior OS in patients who did not have dose reductions or breaks compared with patients who discontinued treatment within the first year. OS outcomes in patients who required dose reductions or breaks but did not discontinue treatment in the first year were similar to those in patients who did not require dose modification. Accepting the limitations of retrospective analysis, these data suggest that patients who are unable to tolerate standard-dose acala or who require treatment breaks may benefit from continuing or resuming treatment at a reduced dose rather than stopping treatment altogether. For patients who do not require dose modifications, ongoing follow-up of the ELEVATE-TN trial suggests durable and incrementally improved OS benefit from the addition of obin to acala.
Disclosures
Follows:Roche: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Lilly: Consultancy, Honoraria, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Centessa: Consultancy, Honoraria, Speakers Bureau; Genesis Care: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria, Speakers Bureau. Ferrajoli:Beigene: Research Funding; AstraZeneca: Honoraria, Research Funding; Abbvie: Honoraria, Research Funding; GenMab: Research Funding; Genetech: Honoraria; Janssen: Honoraria. Byrd:OSU Drug Devel. Inst.: Consultancy; Orbimed: Consultancy, Research Funding; Eilean Therapeutics: Consultancy, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Orange Grove Bio: Membership on an entity's Board of Directors or advisory committees; Vincerx: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Newave: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kurome: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; American Cancer: Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Other: TRAVEL, ACCOMMODATIONS, EXPENSES. Shaw:AstraZeneca: Current Employment. Nozaki:AstraZeneca: Current Employment. Stewart:AstraZeneca: Current Employment. Palhares De Miranda:AstraZeneca: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Sharman:Merck, Novartis: Consultancy; Seattle Genetics: Research Funding; AbbVie, AstraZeneca, BMS, Beigene, Lilly, Genentech, Inc., Genmab: Consultancy; AbbVie, AstraZeneca, BeiGene, BMS, Genentech, Inc., Lilly: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal